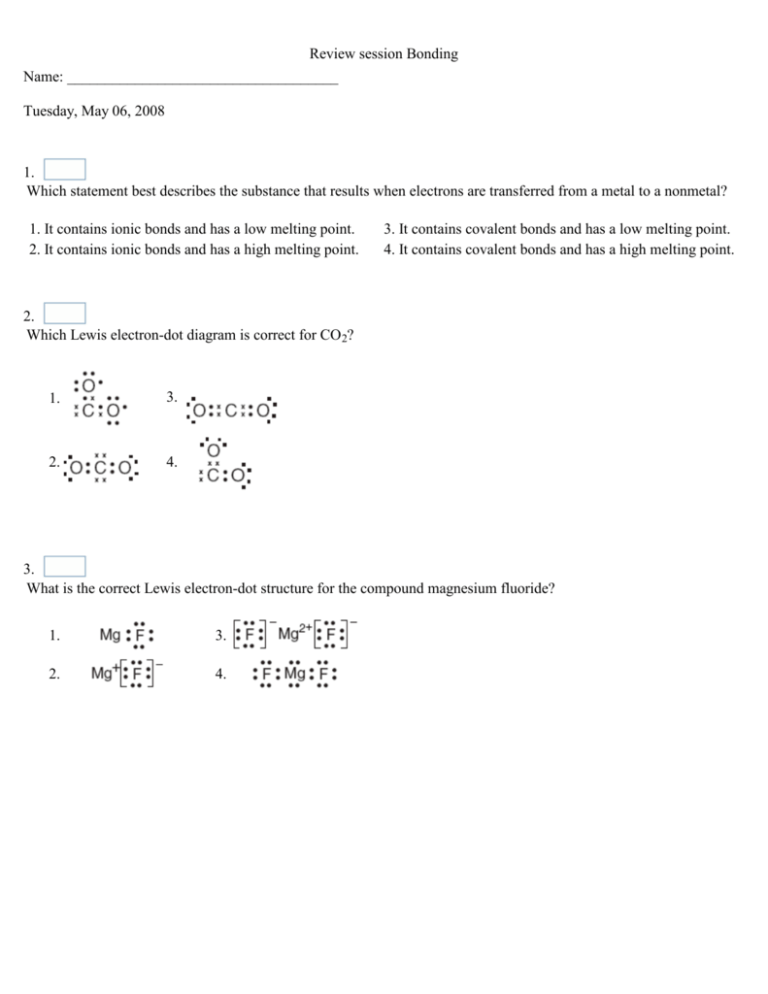
18 Which Best Describes the Bonding in Cu S
NH 4 because nitrogen needs one electron and each hydrogen needs four electrons. Which one best describes the rust found on a vehicle over time.
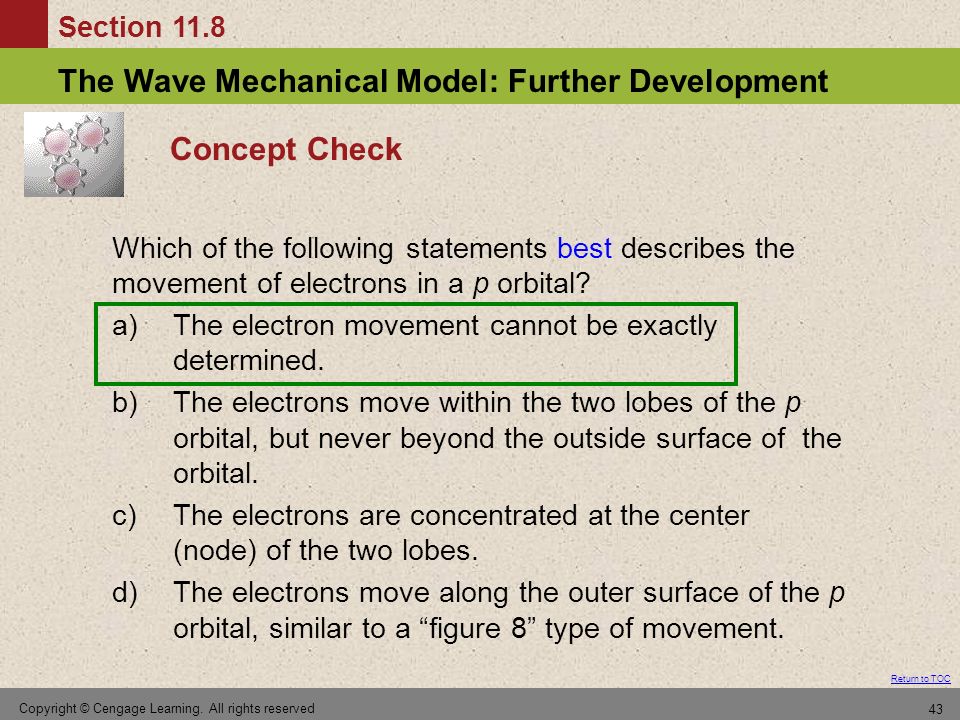
Chapter 11 Modern Atomic Theory Ppt Video Online Download
2 A bond is formed and energy is released.

. Select f Is the OF2 molecule polar or nonpolar. Using the bond energies shown estimate the heat of combustion for one mole of acetylene. The greatest degree of ionic character would be found in a bond between sulfur and A.
When a reaction occurs between atoms with ground state electron con gurations 1s22s1 and 1s22s22p5 the predominant type of bond formed is A. A Mass is the same as weight. Which of the following best describes the type of bonding in a sample of CO2 s answer choices.
Which statement is correct. Electrons are shared and the bonding is ionic. Metals with a body-centered cubic lattice contain a net of four metal atoms per unit cell.
Forces among Br2 molecules are stronger than those among Cl2 molecules. Select e Use electronegativity values and determine if the O-F bond is classified as covalent polar covalent or ionic. C Weight depends on gravity.
Perform the following calculation maintaining the correct number of significant figures. Cus Molar Mass gmol 581 581 Acetone -propanol Butane H H H H H o H H H c H H H H. 2 pts BONUS Questions 1 pt.
The concentration of methane in Earths atmosphere in 1998 expressed as a mole fraction was 1745 nmolmol ppb. The bonding in quartz is best described as. At 1 atom equal masses of H2O s H2O l and H2Og have.
Electrons are shared and the bonding is covalent. Of body centered cube. What is the coordination no.
A The forming of the H-Cl bond releases energy. It is the electrostatic attraction between positive ions and delocalized electrons and occurs by the. 3 A bond is broken and energy is absorbed.
1 A bond is formed and energy is absorbed. A Strong covalent bonds between atoms with similar electronegativities. Z all areas of the US.
Each 33 Given the reaction. 40Base your answer to the following question on Which pair of characteristics describes the molecule illustrated below. Electrons are transferred and the bonding is ionic.
D It is very soluble in water because its. Strong multiple covalent bonds including pi bonds with weak intermolecular forces. Which one of the following describes the major intermolecular force in I2 s.
Asymmetrical and polar Bsymmetrical and nonpolar Casymmetrical and. How many bonding electrons are there in the urea molecule. NH 3 because nitrogen forms a single bond with each hydrogen atom.
B Mass and weight are always proportional to each other. Lattice energy of LiCls -829 kJmol H 2 g -- 2H g bond energy of H 2 432 kJ. A dipole-dipole b London c ionic bonding d hydrogen bonding.
In bonding and nonbonding pairs. Which of the following best describes the type of bonding in. Up to 24 cash back best describes the intermolecular forces of A Benzene is nonpolar.
Atomospheric methane is a potent greenhouse gas. NH3 17034 gmol CuO 7955 gmol N2 2802 gmol Cu 6355 gmol H20 18016 gmol Please. Up to 24 cash back Ahydrogen bonding Bcovalent bonding Cmetallic bonding Dionic bonding 39Which term represents an intermolecular force in a sample of water.
18 MATH Short Answer In the algebra of real-valued functions give the name or number of all of the following 3 choices that the implied domain usually excludes. Apply scientific notations if necessary. S S S S 10 273 273 1 273 1 273 ChemJune 00 2 Part I Answer all 56 questions in this part.
Lower because London dispersion forces among C2h5OH molecules are greater than those among CH3OH molecules. Which of the following best describes the properties of a metallic solid. Ionization energy describes the energy required to remove an.
Z ALL AREAS OF THE US. D S 8 e diamond. Check the book or make your best guess.
At room temperature 12s is a molecular solid. Quartz SiO2 is a solid with a melting point of 1550C. Athe transfer of electrons from one atom to another producing charges which then interact with each other according to Coulombs Law bby sharing electrons in pairs with metal adjacent atoms cby fusion of the atomic nuclei to produce a continuous malleable array.
Which is the weakest intermolecular force among a group of molecules of comparable molecular weight molar mass. Compared to the equilibrium vapor pressure of CH3OH l at 300 K the equilibrium vapor pressure of C2H5OH l at 300 K is. Which of the following best describes the bonding between atoms in metals.
Strong single covalent bonds with weak intermolecular forces. The strongest hydrogen bonding. Which statement best describes ionic bonding.
Up to 24 cash back B. Clg Clg Æ Cl 2 g energy Which statement best describes the reaction. Have nearly constant shallow-ground temperatures.
D Mass depends on gravity. 1s2 2s2 2p6. 2 Which of the following bonds would be best categorized as covalent.
HAVE NEARLY CONSTANT SHALLOW-GROUND TEMPERATURES. NH 4 because nitrogen needs four electrons and each hydrogen needs one electron. PCl 4 ClO 2.
For each statement or question select the word or expression that of those given best completes the statement or answers the question. By 2008 however global methane levels which had stayed mostly flat since 1998 had risen to 1800 nmolmol. Record your answer on the separate answer sheet in accor-.
H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction. 32 Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond. Play this game to review Chemical Bonds.
Electrons are transferred and the bonding is covalent. A lattice of positive and negative ions held together by electrostatic forces. Select d Which best describes the F-O-F bond angles in OF2.
1 selenium 3 oxygen 2 tellurium 4 sulfur 14 Given the reaction. H 3 PO 4. A neutral atom with the electron configuration 1s2 2s2 2p4 would most likely form a bond with an atom having the configuration a.
N-F A I only B II only C III only D I and III E I II and III 3 Which of the following BEST describes the bonding found within solid Al 2 O 3. A soft very low melting point poor electrical conductor. NH 3 because nitrogen forms a triple bond with each hydrogen atom.

Atomic Structure Periodic Table Science Quiz Quizizz

Customer Service Online Social Media Social Media Infographic Infographic
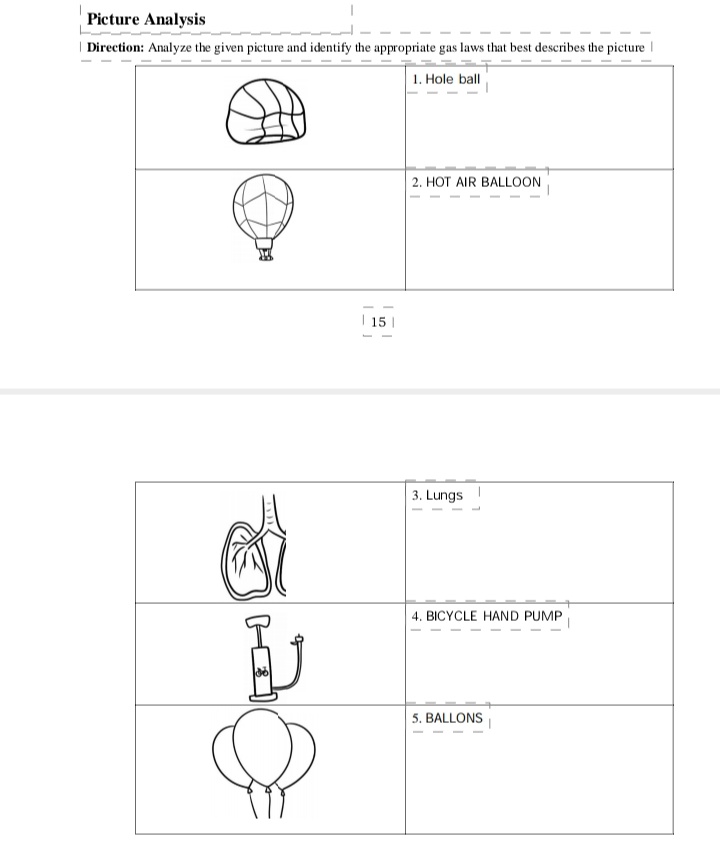
Answered Picture Analysis Direction Analyze Bartleby

Periodic Table 8 P 1 2 Chemistry Quiz Quizizz

Exam Review Which Of The Following Is An Example Of A Mixture Ppt Video Online Download

Invitation Spa Party Invitations Spa Night Girl Spa Party

Covalent Bonding Molecular Compounds Multiple Choice Review

Solved C Dispersion And Hydrogen Bonding D Dispersion And Chegg Com

Quiz Worksheet What Types Of Bonds Can Carbon Form Study Com

Family Quotes Family Quotes Inspirational Quotes Mother Quotes

Possible Multiple Choice Questions

Beauty Isn T About Having A Pretty Face It S About Having A Pretty Mind A Pretty Heart And A Prett Inspirational Quotes Quotable Quotes Inspirational Verses

Free Leadershp Skills Quiz Leadership Skills Leadership Skills

Family Like Branches On A Tree We All Grow In Different Directions Yet Our Roots Remain As One Peace Quotes Family Quotes Inspirational Quotes